Primero hablaremos de Onpattro y en otra entra hablaremos de Tegsedi.
Onpattro (Patisiran) es un medicamento autorizado por la EMA el 27 de agosto de 2018. En su EPAR puede leerse como indicaciones:
4.1 Indicaciones terapéuticas
Onpattro está indicado para el tratamiento de la amiloidosis hereditaria por transtiretina (ATTRh) en pacientes adultos con polineuropatía en estadio 1 o 2.
y como mecanismo de acción:
5.1 Propiedades farmacodinámicas
Grupo farmacoterapéutico: no se ha asignado aún, código ATC: no se ha asignado aún.
Mecanismo de acción:
Onpattro contiene patisirán, un ácido ribonucleico pequeño de interferencia (siRNA) bicatenario que aborda específicamente una secuencia conservada genéticamente en la región 3' no traducida del ARNm de todas las TTR mutantes y naturales. El patisirán está formulado en nanopartículas lipídicas para administrar el siRNA a los hepatocitos, la principal fuente de proteína TTR en la circulación. A través de un proceso natural denominado interferencia de ARN (RNAi), el patisirán produce la degradación catalítica del ARNm de la TTR en el hígado, lo que da lugar a una reducción de la proteína TTR en suero.
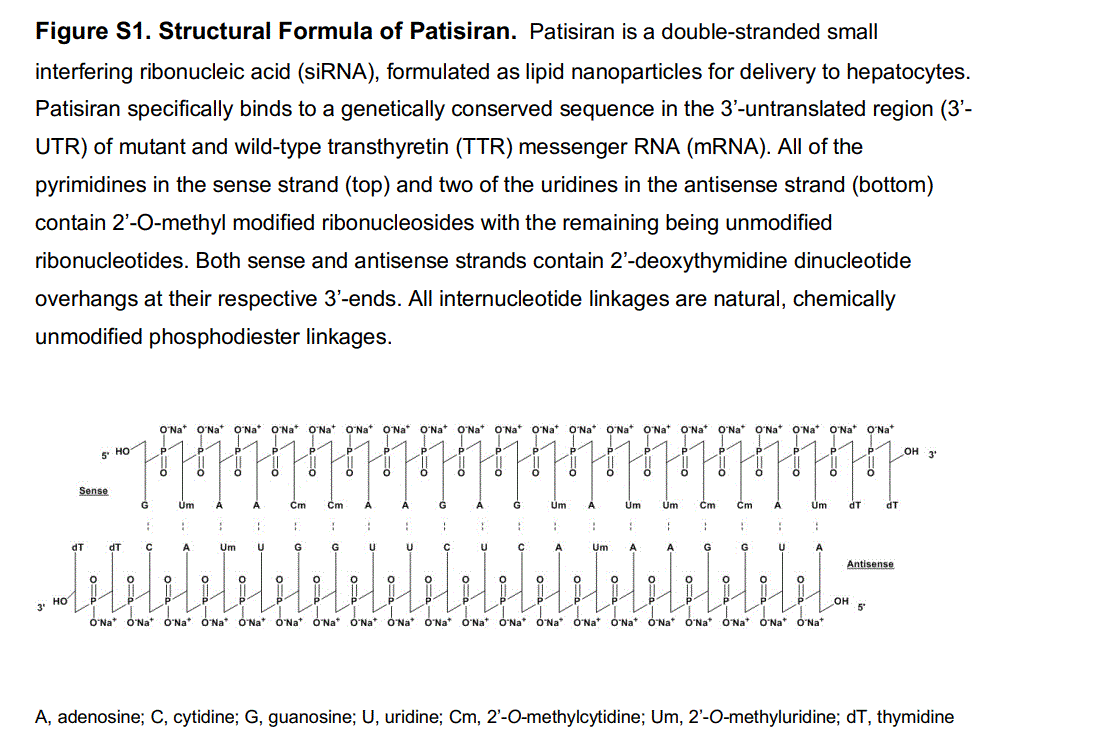
Del artículo Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. presentamos su estructura de 21 por 2 bases.
El titular de la autorización es Alnylam Netherlands B.V
Sobre la etiopatogenia de la amiloidosis hereditaria por transtiretina, en el documento de la EMA, Assessment report dice:
There are more than 120 reported TTR genetic mutations associated with hATTR amyloidosis, and almost all patients are heterozygous for the mutated TTR allele. The most common genotype is the valine to methionine mutation at position 30 (V30M), accounting for approximately 50% of cases worldwide, and occurring primarily in families with heritage from Portugal, Sweden, Japan, and Brazil.(9) Mutations in the TTR gene lead to destabilization of the tetrameric protein and disassociation of the TTR subunits into dimers and individual mutant and wt monomers, which subsequently misfold. These misfolded TTR monomers can then self-assemble into oligomers and form amyloid fibrils and plaques in the extracellular space of various tissues, including the peripheral nervous system, heart, gastrointestinal tract, kidney, central nervous system (CNS) and eye leading to cellular injury and organ dysfunction with corresponding clinical manifestations.
Por otro lado, respecto a si estamos ante un ATMP, la EMA define un ATMP como:
gene therapy medicines:
these contain genes that lead to a therapeutic, prophylactic or diagnostic effect. They work by inserting 'recombinant' genes into the body, usually to treat a variety of diseases, including genetic disorders, cancer or long-term diseases. A recombinant gene is a stretch of DNA that is created in the laboratory, bringing together DNA from different sources;
una terapia celular está claro que no es.
Se trata de un medicamento que actúa a nivel citoplasmático produciendo la degradación catalítica del mARN sin incorporar material recombianante en el ADN del paciente, por lo que el medicamento intervendría en la traducción del ADN no en su composición, no siendo por ello un ATMP.
El más importante CT de fase III es APOLLO y sus resultados:
A total of 225 patients underwent randomization (148 to the patisiran group and 77 to the placebo group). The mean (±SD) mNIS+7 at baseline was 80.9±41.5 in the patisiran group and 74.6±37.0 in the placebo group; the least-squares mean (±SE) change from baseline was −6.0±1.7 versus 28.0±2.6 (difference, −34.0 points; P<0.001) at 18 months. The mean (±SD) baseline Norfolk QOL-DN score was 59.6±28.2 in the patisiran group and 55.5±24.3 in the placebo group; the least-squares mean (±SE) change from baseline was −6.7±1.8 versus 14.4±2.7 (difference, −21.1 points; P<0.001) at 18 months. Patisiran also showed an effect on gait speed and modified BMI. At 18 months, the least-squares mean change from baseline in gait speed was 0.08±0.02 m per second with patisiran versus −0.24±0.04 m per second with placebo (difference, 0.31 m per second; P<0.001), and the least-squares mean change from baseline in the modified BMI was −3.7±9.6 versus −119.4±14.5 (difference, 115.7; P<0.001). Approximately 20% of the patients who received patisiran and 10% of those who received placebo had mild or moderate infusion-related reactions; the overall incidence and types of adverse events were similar in the two groups.
Como viene sucediendo con los medicamentos resultado de importante investigación genética su precio es muy importante: 345.000$ y en la tabla de Mickle, K et al. se puede ver el coste de los QALY
y la conclusion del estudio:
The RNA-based therapeutics patisiran and inotersen for hATTR represent an important advance in the treatment of a rare genetic disorder with high unmet need. However, despite consideration of potential broader benefits and of the contextual considerations associated with treatment of an ultra-rare disorder, the pricing of these drugs in the U.S. health care system was judged to represent low long-term value for money. Further efforts are needed to help align the price of these treatments with their demonstrated benefits in order to ensure sustainable access to high-value care for all patients
Le ruego evalúe la presente entrada asignando un valor de 1 a 5, de peor a mejor valorada, usando la opción de comentarios
Onpattro (Patisiran) es un medicamento autorizado por la EMA el 27 de agosto de 2018. En su EPAR puede leerse como indicaciones:
4.1 Indicaciones terapéuticas
Onpattro está indicado para el tratamiento de la amiloidosis hereditaria por transtiretina (ATTRh) en pacientes adultos con polineuropatía en estadio 1 o 2.
y como mecanismo de acción:
5.1 Propiedades farmacodinámicas
Grupo farmacoterapéutico: no se ha asignado aún, código ATC: no se ha asignado aún.
Mecanismo de acción:
Onpattro contiene patisirán, un ácido ribonucleico pequeño de interferencia (siRNA) bicatenario que aborda específicamente una secuencia conservada genéticamente en la región 3' no traducida del ARNm de todas las TTR mutantes y naturales. El patisirán está formulado en nanopartículas lipídicas para administrar el siRNA a los hepatocitos, la principal fuente de proteína TTR en la circulación. A través de un proceso natural denominado interferencia de ARN (RNAi), el patisirán produce la degradación catalítica del ARNm de la TTR en el hígado, lo que da lugar a una reducción de la proteína TTR en suero.
Del artículo Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. presentamos su estructura de 21 por 2 bases.
El titular de la autorización es Alnylam Netherlands B.V
Sobre la etiopatogenia de la amiloidosis hereditaria por transtiretina, en el documento de la EMA, Assessment report dice:
There are more than 120 reported TTR genetic mutations associated with hATTR amyloidosis, and almost all patients are heterozygous for the mutated TTR allele. The most common genotype is the valine to methionine mutation at position 30 (V30M), accounting for approximately 50% of cases worldwide, and occurring primarily in families with heritage from Portugal, Sweden, Japan, and Brazil.(9) Mutations in the TTR gene lead to destabilization of the tetrameric protein and disassociation of the TTR subunits into dimers and individual mutant and wt monomers, which subsequently misfold. These misfolded TTR monomers can then self-assemble into oligomers and form amyloid fibrils and plaques in the extracellular space of various tissues, including the peripheral nervous system, heart, gastrointestinal tract, kidney, central nervous system (CNS) and eye leading to cellular injury and organ dysfunction with corresponding clinical manifestations.
Por otro lado, respecto a si estamos ante un ATMP, la EMA define un ATMP como:
gene therapy medicines:
these contain genes that lead to a therapeutic, prophylactic or diagnostic effect. They work by inserting 'recombinant' genes into the body, usually to treat a variety of diseases, including genetic disorders, cancer or long-term diseases. A recombinant gene is a stretch of DNA that is created in the laboratory, bringing together DNA from different sources;
una terapia celular está claro que no es.
Se trata de un medicamento que actúa a nivel citoplasmático produciendo la degradación catalítica del mARN sin incorporar material recombianante en el ADN del paciente, por lo que el medicamento intervendría en la traducción del ADN no en su composición, no siendo por ello un ATMP.
El más importante CT de fase III es APOLLO y sus resultados:
A total of 225 patients underwent randomization (148 to the patisiran group and 77 to the placebo group). The mean (±SD) mNIS+7 at baseline was 80.9±41.5 in the patisiran group and 74.6±37.0 in the placebo group; the least-squares mean (±SE) change from baseline was −6.0±1.7 versus 28.0±2.6 (difference, −34.0 points; P<0.001) at 18 months. The mean (±SD) baseline Norfolk QOL-DN score was 59.6±28.2 in the patisiran group and 55.5±24.3 in the placebo group; the least-squares mean (±SE) change from baseline was −6.7±1.8 versus 14.4±2.7 (difference, −21.1 points; P<0.001) at 18 months. Patisiran also showed an effect on gait speed and modified BMI. At 18 months, the least-squares mean change from baseline in gait speed was 0.08±0.02 m per second with patisiran versus −0.24±0.04 m per second with placebo (difference, 0.31 m per second; P<0.001), and the least-squares mean change from baseline in the modified BMI was −3.7±9.6 versus −119.4±14.5 (difference, 115.7; P<0.001). Approximately 20% of the patients who received patisiran and 10% of those who received placebo had mild or moderate infusion-related reactions; the overall incidence and types of adverse events were similar in the two groups.
Como viene sucediendo con los medicamentos resultado de importante investigación genética su precio es muy importante: 345.000$ y en la tabla de Mickle, K et al. se puede ver el coste de los QALY
y la conclusion del estudio:
The RNA-based therapeutics patisiran and inotersen for hATTR represent an important advance in the treatment of a rare genetic disorder with high unmet need. However, despite consideration of potential broader benefits and of the contextual considerations associated with treatment of an ultra-rare disorder, the pricing of these drugs in the U.S. health care system was judged to represent low long-term value for money. Further efforts are needed to help align the price of these treatments with their demonstrated benefits in order to ensure sustainable access to high-value care for all patients
Le ruego evalúe la presente entrada asignando un valor de 1 a 5, de peor a mejor valorada, usando la opción de comentarios

5
ResponderEliminarGracias Dra Sánchez
ResponderEliminar